Page 17 - Demo
P. 17
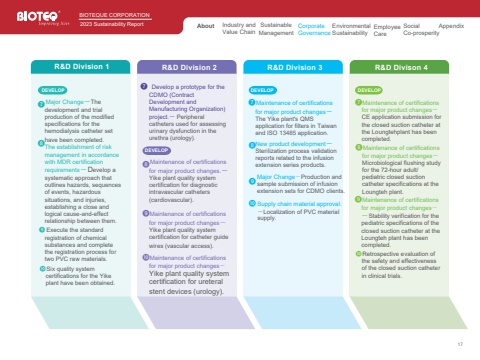
17R&D Division 1DEVELOP%u277c%u277dMajor Change%uff0dThedevelopment and trial production of the modified specifications for the hemodialysis catheter set have been completed.The establishment of risk management in accordance with MDR certification requirements%uff0dDevelop asystematic approach that outlines hazards, sequences of events, hazardous situations, and injuries, establishing a close and logical cause-and-effect relationship between them.%u277e Execute the standardregistration of chemicalsubstances and completethe registration process fortwo PVC raw materials.%u277fSix quality systemcertifications for the Yikeplant have been obtained.R&D Division 2%u277c Develop a prototype for the CDMO (ContractDevelopment andManufacturing Organization)project.%uff0d Peripheralcatheters used for assessingurinary dysfunction in theurethra (urology).%u277dMaintenance of certificationsfor major product changes.%uff0dYike plant quality systemcertification for diagnosticintravascular catheters(cardiovascular).%u277eMaintenance of certificationsfor major product changes%uff0dYike plant quality systemcertification for catheter guidewires (vascular access).%u277fMaintenance of certificationsfor major product changes%uff0dYike plant quality systemcertification for ureteralstent devices (urology).R&D Division 3%u277cMaintenance of certificationsfor major product changes%uff0dThe Yike plant's QMSapplication for filters in Taiwanand ISO 13485 application.%u277dNew product development%uff0dSterilization process validationreports related to the infusionextension series products.%u277e%u277fR&D Divison 4%u277cMaintenance of certificationsfor major product changes%uff0dCE application submission forthe closed suction catheter atthe Loungtehplant has beencompleted.%u277dMaintenance of certificationsfor major product changes%uff0dMicrobiological flushing studyfor the 72-hour adult/pediatric closed suctioncatheter specifications at theLoungteh plant.DEVELOP DEVELOPMajor Change%uff0dProduction andsample submission of infusionextension sets for CDMO clients.Supply chain material approval.%uff0dLocalization of PVC materialsupply.%u277eMaintenance of certificationsfor major product changes%uff0d%uff0dStability verification for thepediatric specifications of theclosed suction catheter at theLoungteh plant has beencompleted.%u277fRetrospective evaluation ofthe safety and effectivenessof the closed suction catheterin clinical trials.BBIOTEQUE CORPORATION2023 Sustainability Report Industry andValue Chain Sustainable ManagementCorporate GovernanceEnvironmental SustainabilityEmployee CareSocial Co-prosperityAbout AppendixDEVELOP