Company Profile
A Life Commitment to Human Being
BIOTEQUE CORPORATION is a leading medical device manufacturer in Taiwan,
specialized in medical disposables for use in hemodialysis access , endovascular
treatment and other fields.
It was founded in 1991 and has become public listed company in Taipei Exchange(TPEx)
since 2002.
BIOTEUQE is currently registered as a device manufacturer, with headquarter being located in Taipei, BIOTEQUE and its affiliates employ over 1,100 employees and currently operates two manufacturing facilities in both Taiwan and Philippines, the land occupation over 100,000 square meters, both equipped with state-of-the-art facilities and accredited with ISO 13485 and compliant with FDA, GMP and Quality System Regulation.

In pursuit of extensive and everlasting growth, BIOTEQUE broke ground on its third manufacturing facility in Yilan Industrial Park on December, 2021. This capacity expansion will offer comprehensive Contract Development and Manufacturing Organization (CDMO) capabilities for contract partners.
BIOTEQUE is continuously dedicated to develop innovative products and provide total solutions for all its customers.
The Quality Assurance System is certified and correspondent with ISO-13485, QMS (GMP) standard. Products are certified with CE mark.
In addition to the current product lines, Bioteque is currently working on several projects to develop new products, like advanced silicone and TPU catheters intended for long-term implantation. We currently have over 21 patents to our credit which cover all areas in medical field. In the meantime, Bioteque is seeking for potential long-term partnership with renowned and established national research organization to develop innovative medical products.

- Company culture
- Integrity Diligence
- Managerial philosophy
- Assured quality, innovative service, speed and cost, as well as team work and mutual prosperity.
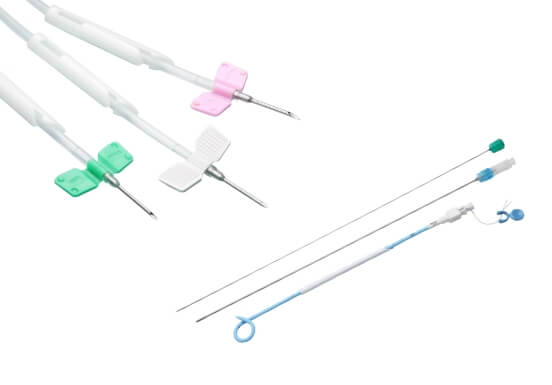
- Blood tubing line, AVF needle, Transducer protector, on line HDF with check valve or without check valve.
- IV infusion bags, Precision IV infusion set, Drainage bag, Insufflation Tubing set w/filter and various surgical drainage tube.
- Full range of medical components which cover blood tubing line components, percutaneous drainage components, infusion bag components, AVF needle components, Precision IV infusion set components and IV infusion bag components….etc.
- Full range of TPU(Thermoplastic polyurethane) catheters includes Pigtail Drainage Catheter Set, Double pigtail ureteral stent set, Biliary Drainage Catheter ,PCN KIT (Percutaneous Nephrostomy Kit), Dialysis catheter Kit.
- Other medical disposables products such as closed suction catheter,….etc.
- Full range of endovascular products include: PTA(Percutaneous Transluminal Angioplasty), Angiography Catheter, Sheath Introducer, CT/MRI Angiography syringes, micro catheter, hydrophilic coated guidewire.

- Class 10,000 clean room & assembly lines
- Automatic assembly machines for Hemodialysis Blood Line Set, AV Fistula Needle, IV bag and Components
- E.O. sterilization equipment
- Advanced Extrusion machines & injection machines
- Molding house
- In house laboratory
- Warehouse facilities

Mission
- Improving Lives

Vision
- Sustainable operation, to be a global leading brand.
- Perpetual innovation, to offer a total solution for all medical needs.
- Mutual prosperity, to share corporate success among all partners.

Quality Policy
- To continuously improve product quality and service
- To endlessly pursue customer’s satisfaction

Management Concept
- Safety, Quality, Service, Innovation, Efficiency, Cost and Team Work
Milestones
- 1991.11
- Bioteque Corporation was authorized to establish with registered capital amounted to NT$30,000,000, paid-in capital NT$30,000,000. Address is located in Taipei.
- 1995.02
- Obtained product license registration from DOH.
- 1998.08
- The initial manufacturer which developed IV infusion bag successfully in Taiwan.
- 1998.09
- ISO-9001 Quality System Accreditated by International Standards Organization.
- 1999.05
- CE Certified.medical equipment manufacturing quality assurance by EU body.
- 1999.12
- Obtained FDA 510(K) clearance to the U.S. market.
- 2003.10
- Bioteque Corp. acquired National Award of Outstanding SMEs
- 2013.01
- Bioteque Corp. acquired Taiwan Mittelstand Award
- 2019.07
- Bioteque Corp. acquired Outstanding Company of the Year from Bio Asia-Taiwan
- 2020.12
- Bioteque Corp. started building the flagship facility in Yilan Science Park.
